INDICATIONS
The CUSTOMFLEX® ARTIFICIALIRIS is intended for use as an iris prosthesis for the treatment of iris defects. The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use in children and adults for the treatment of full or partial aniridia resulting from congenital aniridia, acquired defects, or other conditions associated with full or partial aniridia. When implanted, the CUSTOMFLEX® ARTIFICIALIRIS mimics the natural iris and produces an improvement in the visual symptoms associated with aniridia that most affect the patient's quality of life.
These symptoms include:
Starburts
Glare and halos day and night
Light sensitivity day and night
Reading difficulty
Difficulty driving at night
Documents
Other Resources
This page has important information to help you decide whether to have CUSTOMFLEX® ARTIFICIALIRIS surgery to repair your iris defect and improve the quality and quantity of your daily living.
Please read this page completely and discuss the risks and benefits with your eye care professional. Your doctor can help you decide if the CUSTOMFLEX® ARTIFICIALIRIS is suitable for you. Discuss the content of this page and any questions you may have with your doctor. Check with your doctor if any of the possible contraindications, precautions and warnings may apply to you. Make sure your doctor answers all your questions to your satisfaction before you agree to have the CUSTOMFLEX® ARTIFICIALIRIS treatment
EXAMPLES
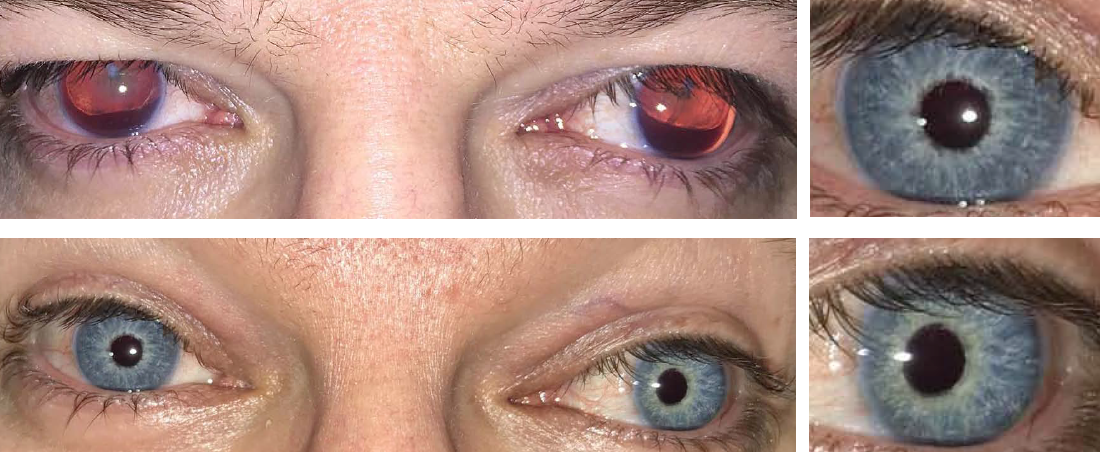
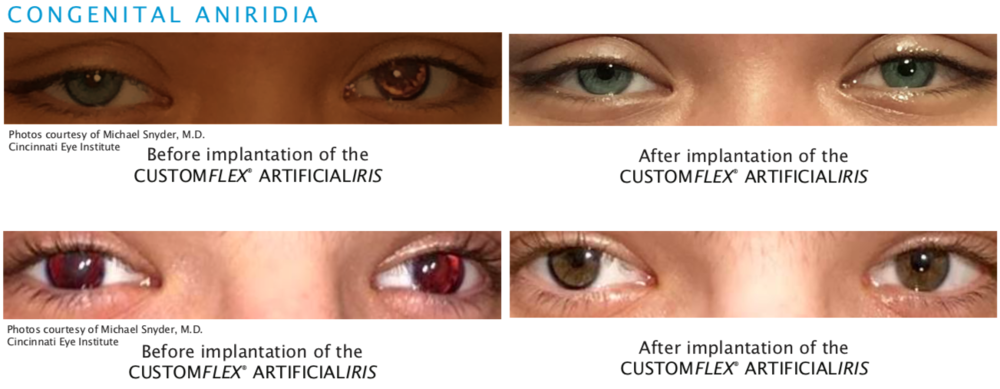
Congenital Aniridia
Congenital aniridia is a rare genetic disorder characterized by a complete or partial absence of the iris.
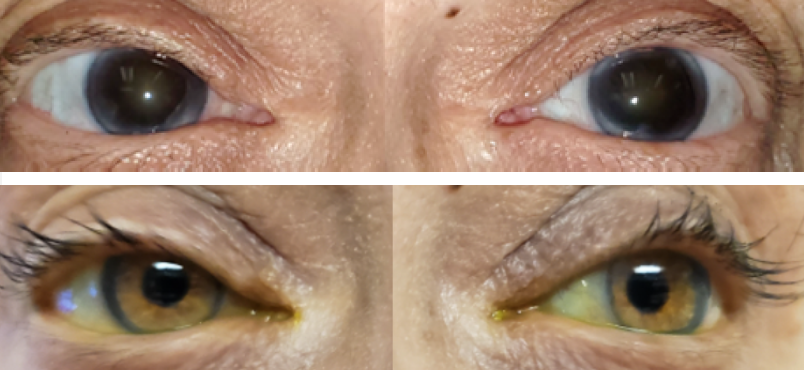
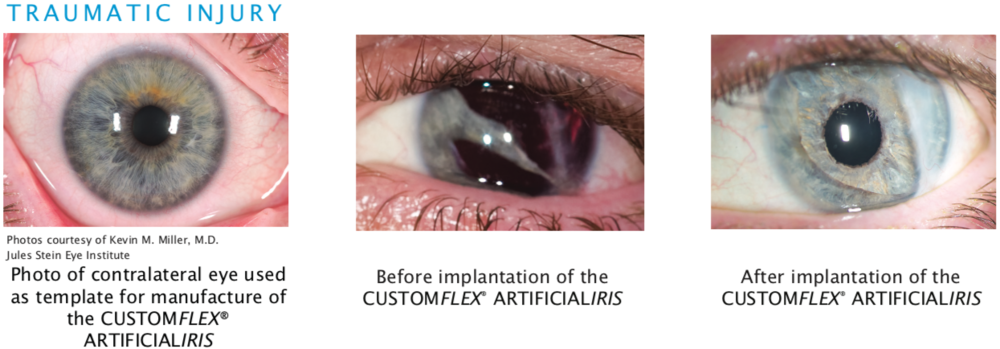
Traumatic Injury
Aniridia acquired as the result of an accident or some other type of trauma or injury to the eye.
Oculocutaneous Albinism
Full or partial aniridia can be associated with conditions such as ocular or oculocutaneous albinism.
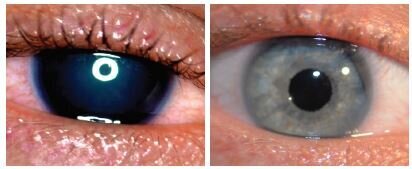
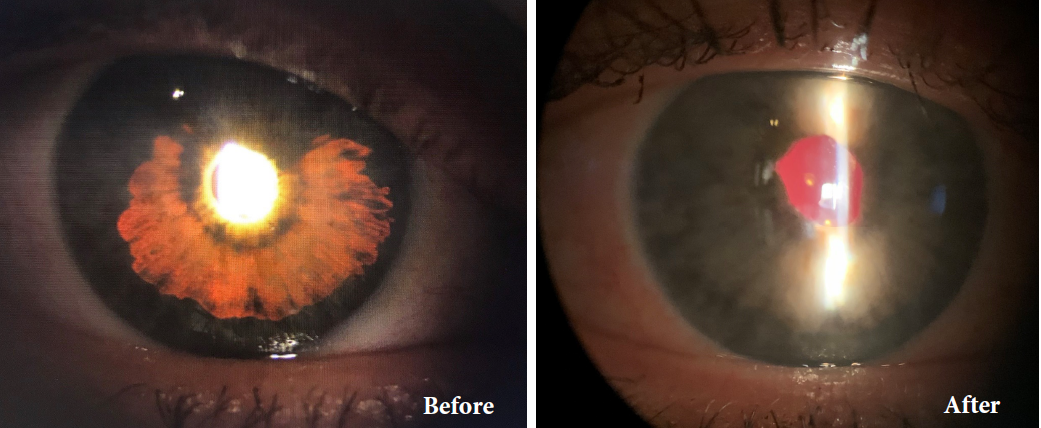
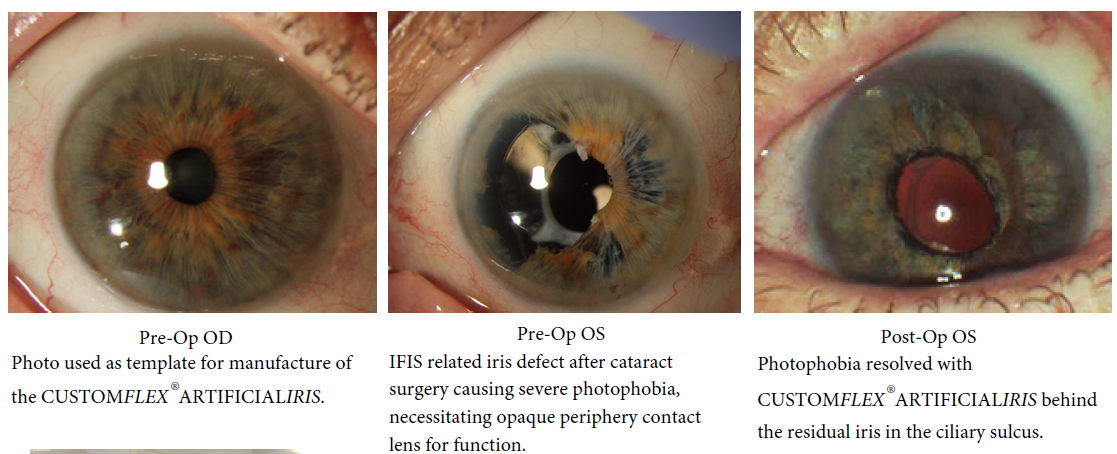
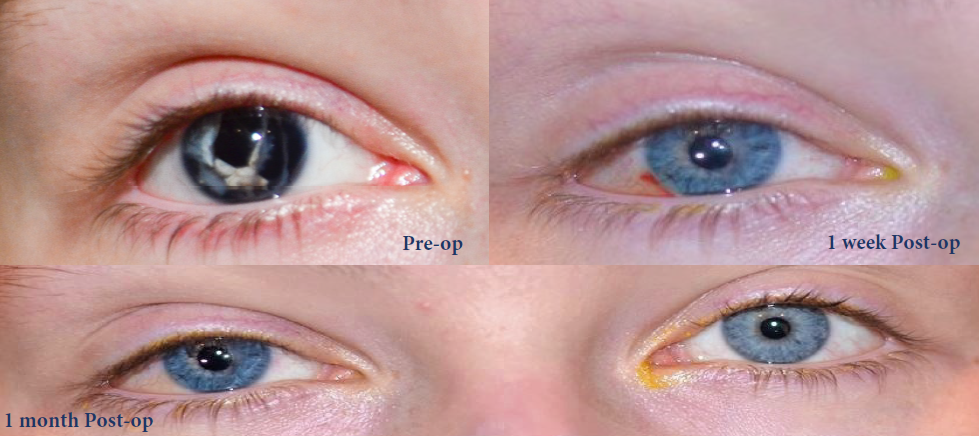
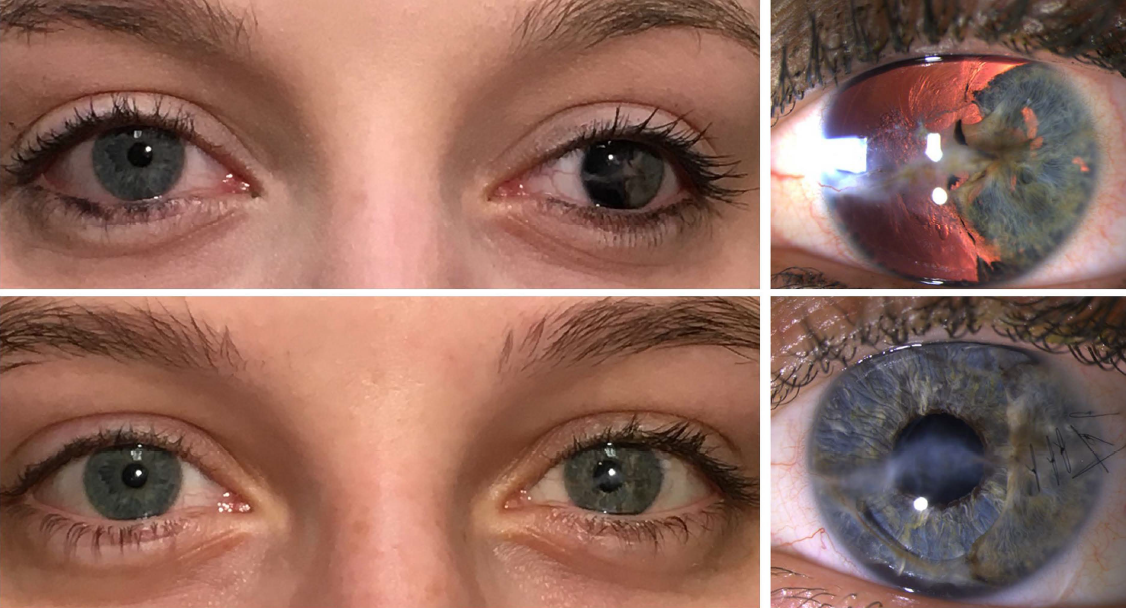
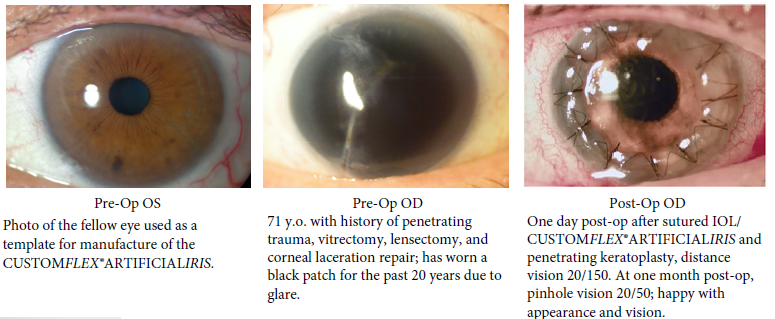
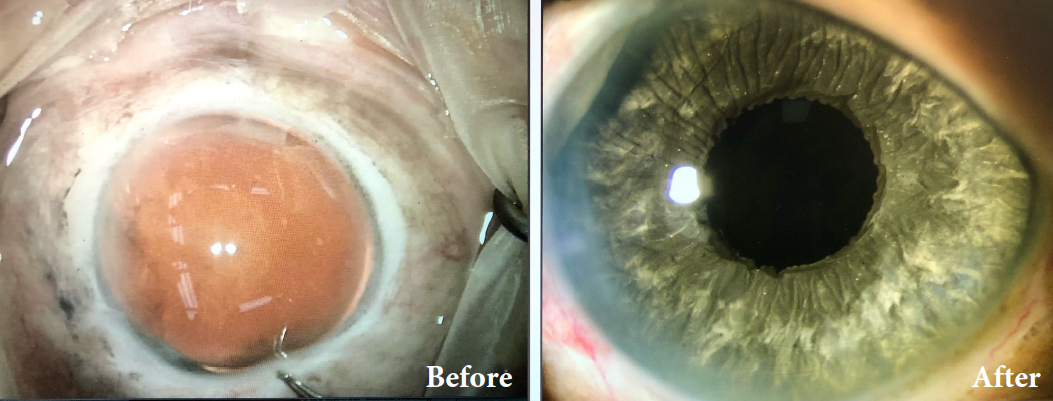
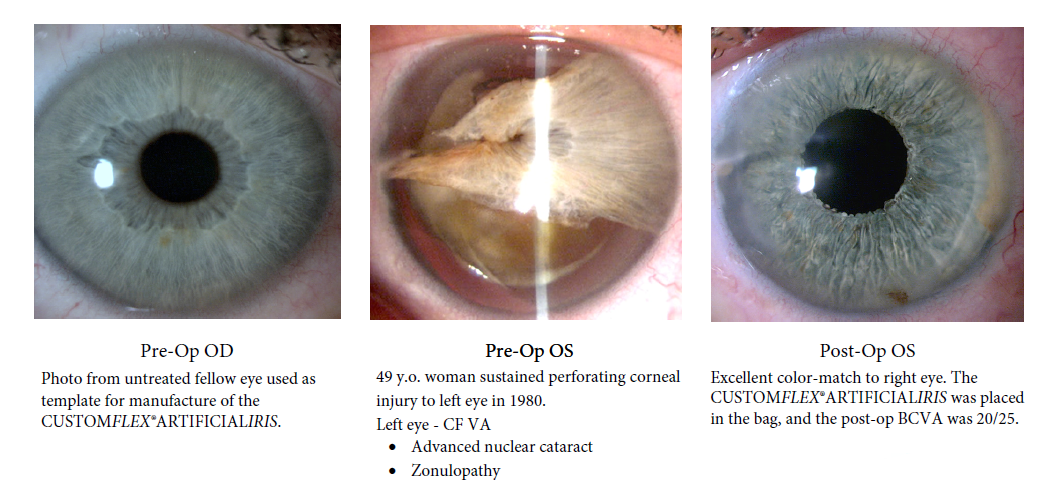
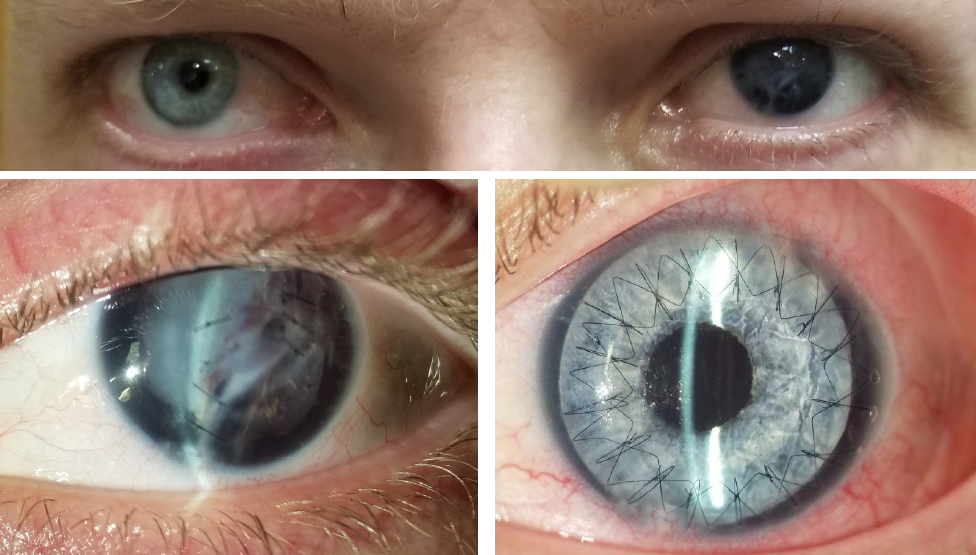
Before and after implantation of the CUSTOMFLEX® ARTIFICIALIRIS.
CUSTOM-COLOR MATCH
Every CUSTOMFLEX® ARTIFICIALIRIS is custom-made to match the patient's natural iris tissue, addressing both symptomatic and cosmetic aspects of iris defects.
Find a Certified Surgeon Near You
VEO Ophthalmics offers a free two-part Training Program and self certification for US surgeons to become certified to implant the CUSTOMFLEX® ARTIFICIALIRIS.
CLINICAL SCIENCE
The safety and efficacy of the CUSTOMFLEX® ARTIFICIALIRIS was demonstrated in a non-randomized clinical trial of 447 eyes of 389 adult and pediatric patients with aniridia or other iris defects.
Benefits:
There was a decrease in the severity of day-time symptoms of light sensitivity, glare and halos.
There was a decrease in the severity of night-time symptoms of light sensitivity, glare, halos and starbursts.
Reading was improved.
Difficulty driving at night was improved.
There was a three-fold improvement in patients' ability to complete normal vision-related activities of daily living, as measured by a standardized health related quality of life questionnaire.
Satisfaction with the cosmetic appearance was high, with 93.8% of patients rating their appearance as “improved” to “very much improved” after implantation of the CUSTOMFLEX® ARTIFICIALIRIS.
Although the CUSTOMFLEX® ARTIFICIALIRIS is not designed to improve vision, 67.2% of the eyes had better uncorrected visual acuity (vision without glasses or contact lenses) after the artificial iris surgery; and, 27.7% of the eyes had uncorrected vision that was unchanged.
Risks:
None of the eyes had a clinically significant loss of more than 2 lines on the eye chart of best corrected vision at 12 months postoperatively that was caused by the CUSTOMFLEX® ARTIFICIALIRIS.
Increases in intraocular pressure greater than 30 mm Hg were the most common adverse event reported in the study.
The CUSTOMFLEX® ARTIFICIALIRIS implant remained in its intended position in the majority of eyes throughout the 12 month study; secondary surgeries could be performed when needed to reposition the device.
Inflammation occurring at one month or later after surgery was related to the CUSTOMFLEX® ARTIFICIALIRIS device in 0.7% of the eyes.
Contraindications: CUSTOMFLEX® ARTIFICIALIRIS is contraindicated for patients with certain eye conditions, such as uncontrolled inflammation, severe chronic uveitis, microphthalmus, untreated retinal detachment, untreated chronic glaucoma, rubella cataract, rubeosis of the iris, proliferative diabetic retinopathy, Stargardt’s retinopathy, or intraocular infections, or in pregnant women.
Warnings: Implantation of the CUSTOMFLEX® ARTIFICIALIRIS is not recommended in children who are less than 3 years of age because their eyes are still in a stage of major growth development that would be disrupted by ocular surgery. Refer to the Patient Information Brochure for complete study results, other warnings, precautions, and instructions for the use of the device.