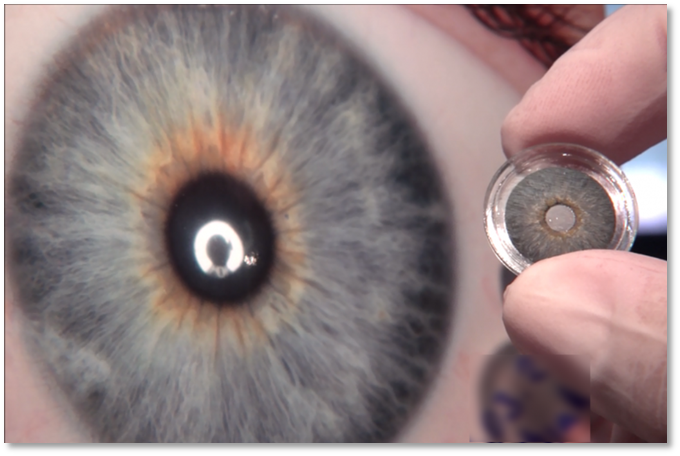
CUSTOMFLEX® ARTIFICIALIRIS FDA Approved and Available in the U.S.
Recipient of first Breakthrough Medical Device approval issued by FDA
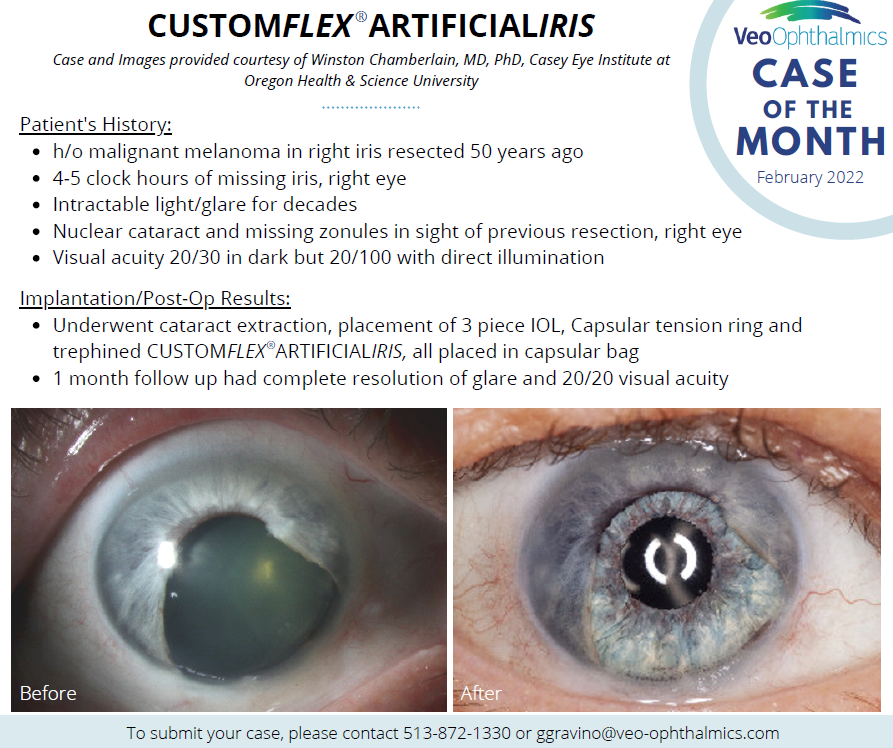
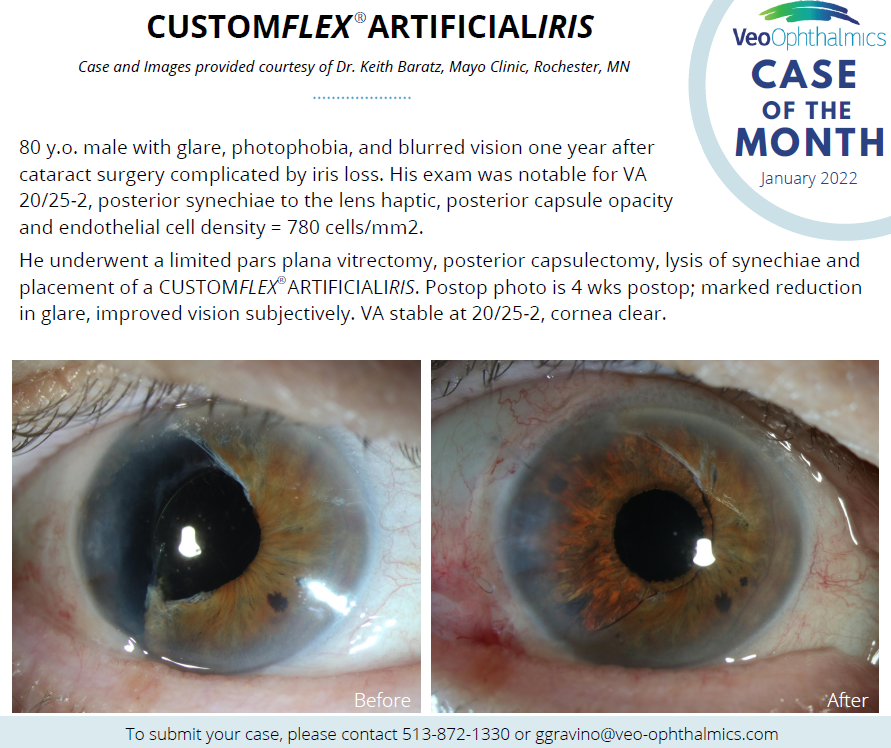
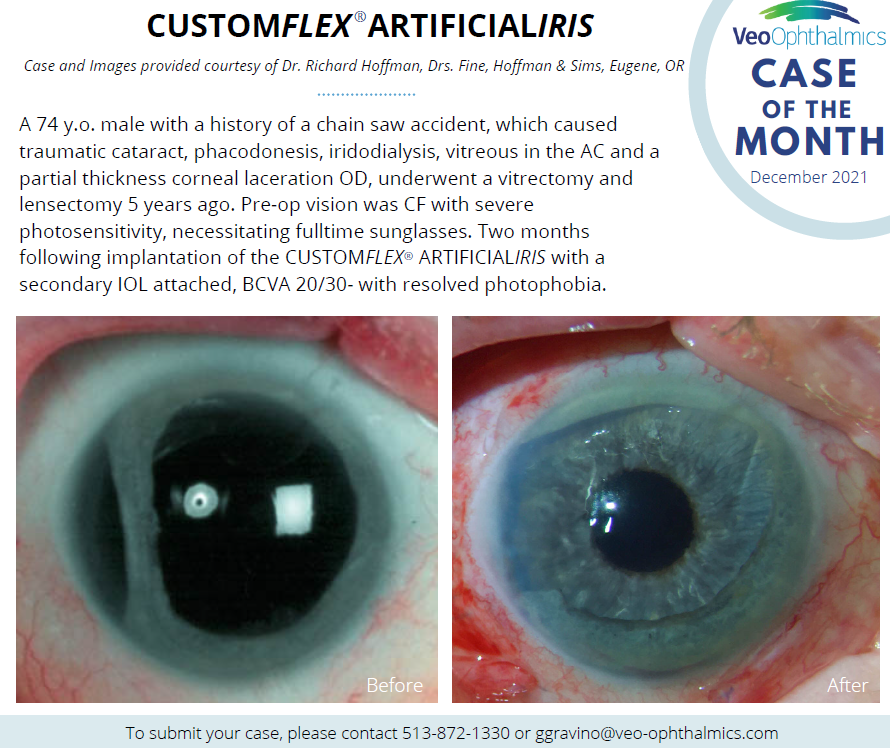
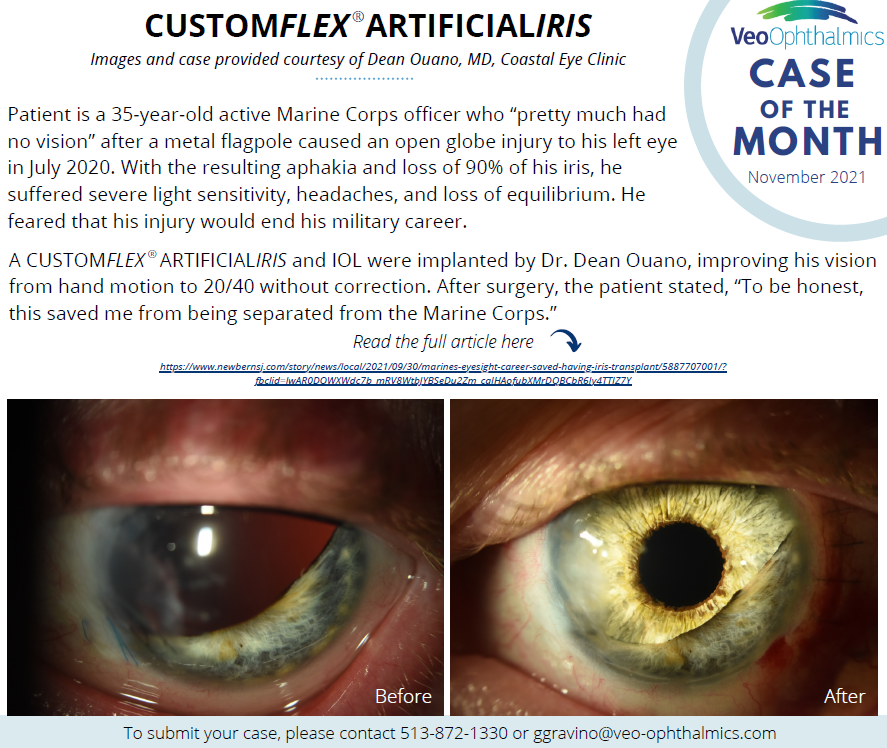
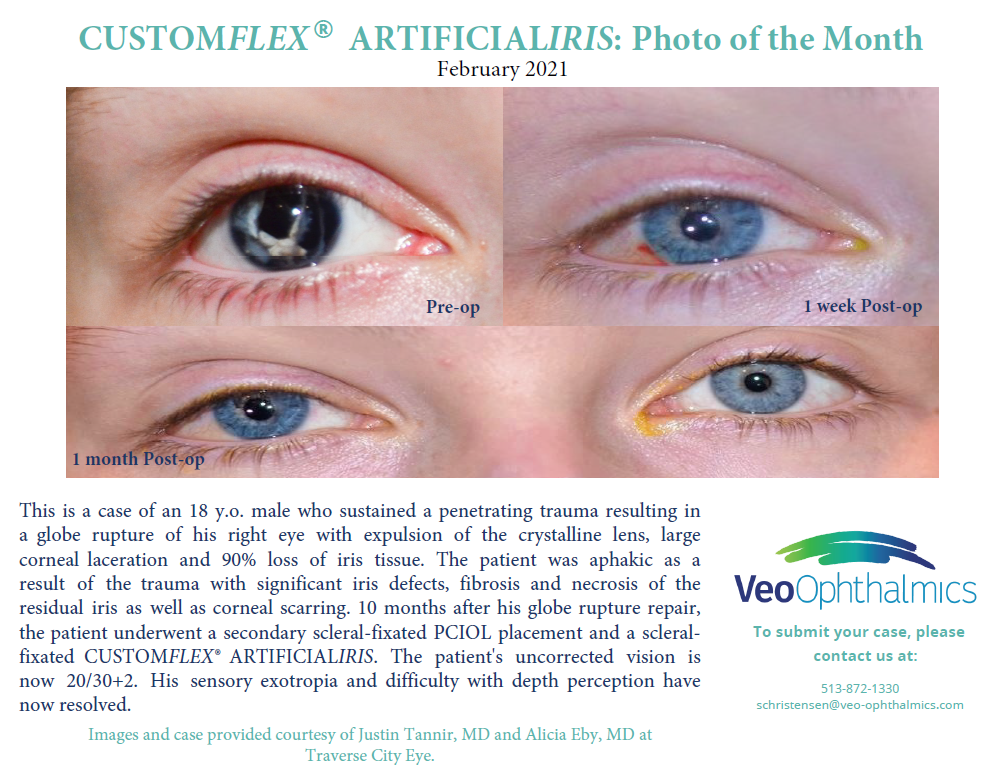
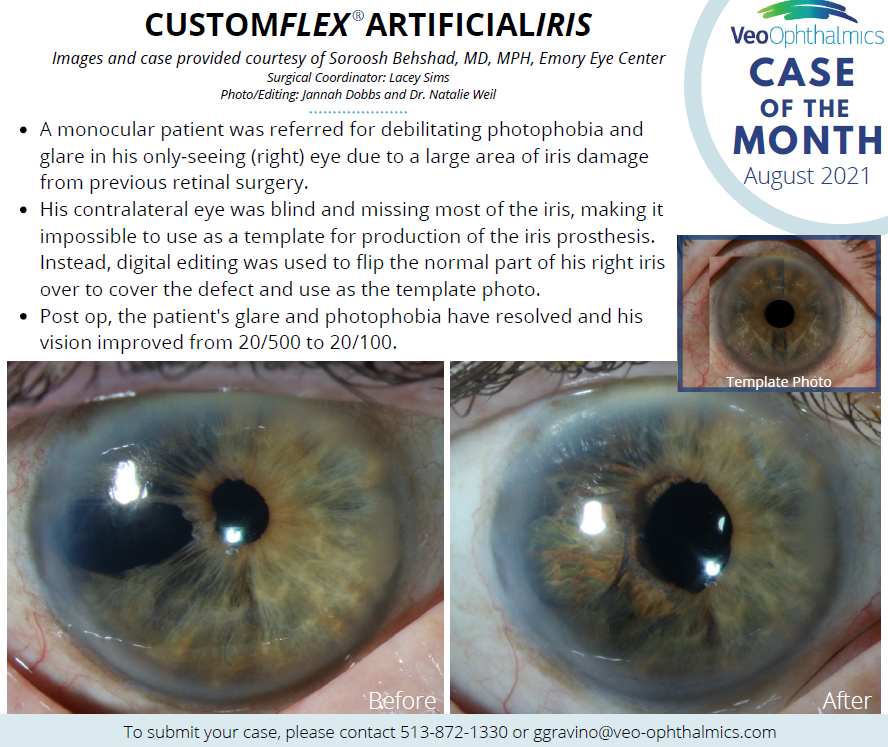
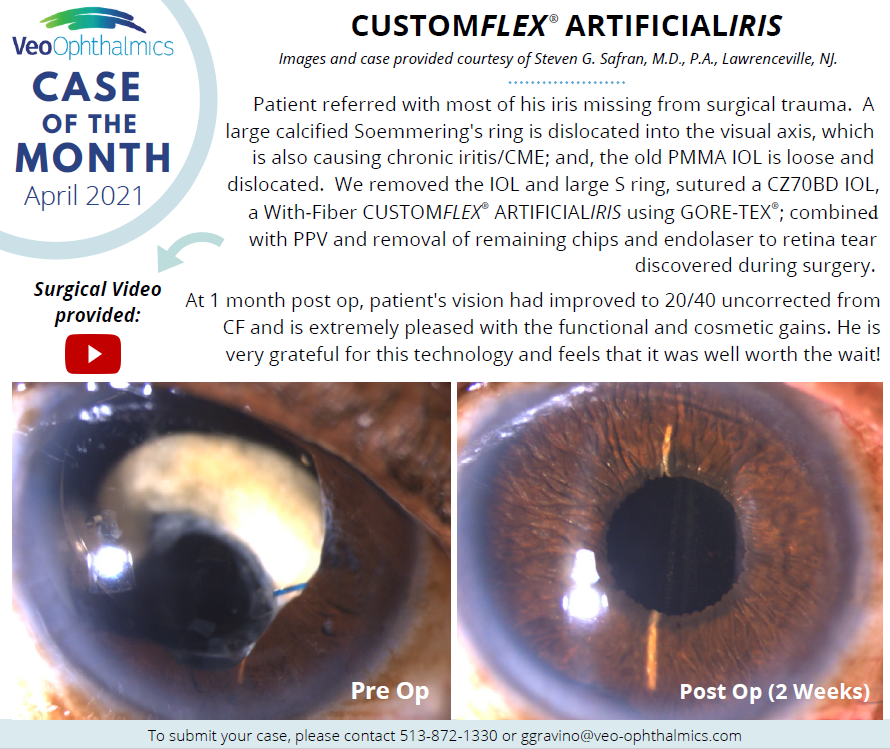
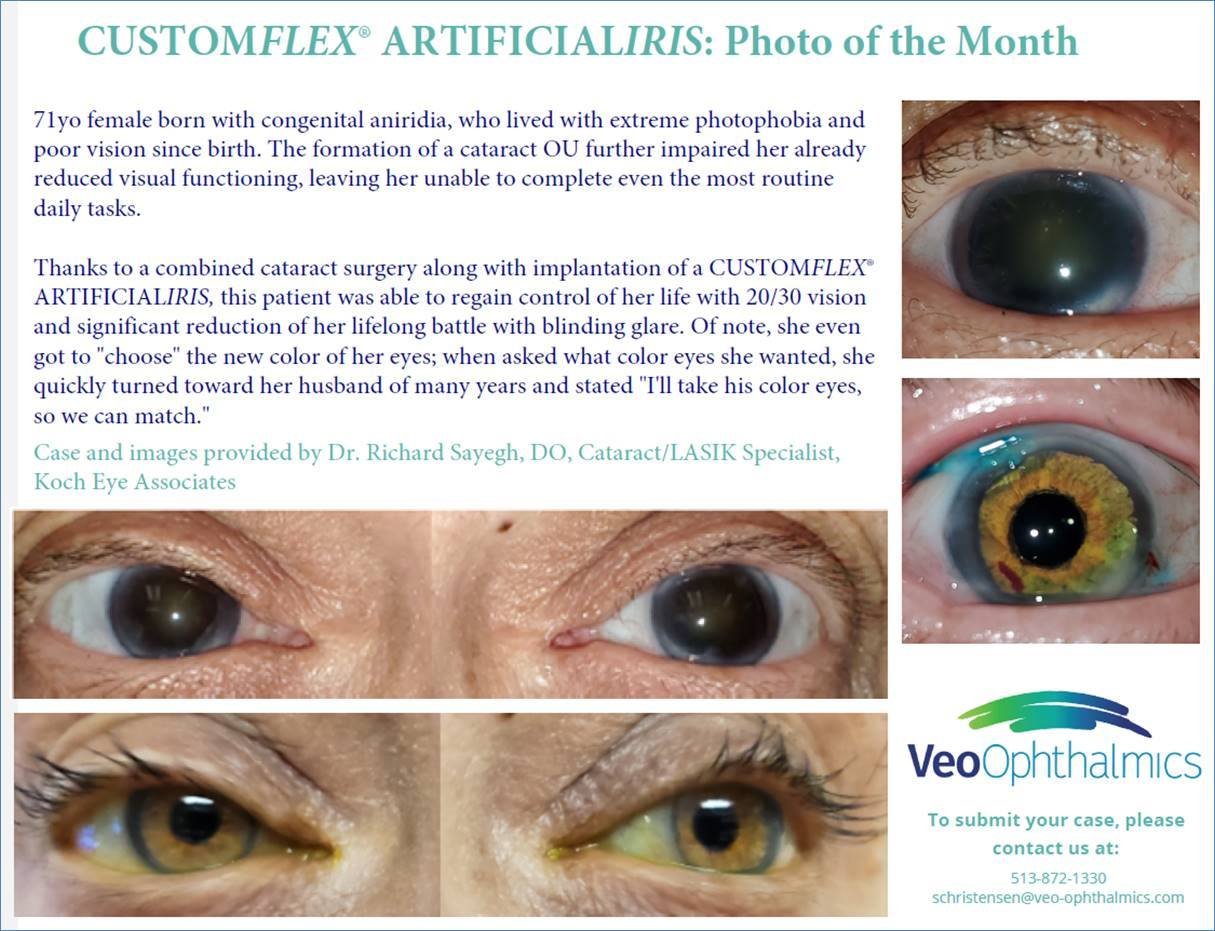
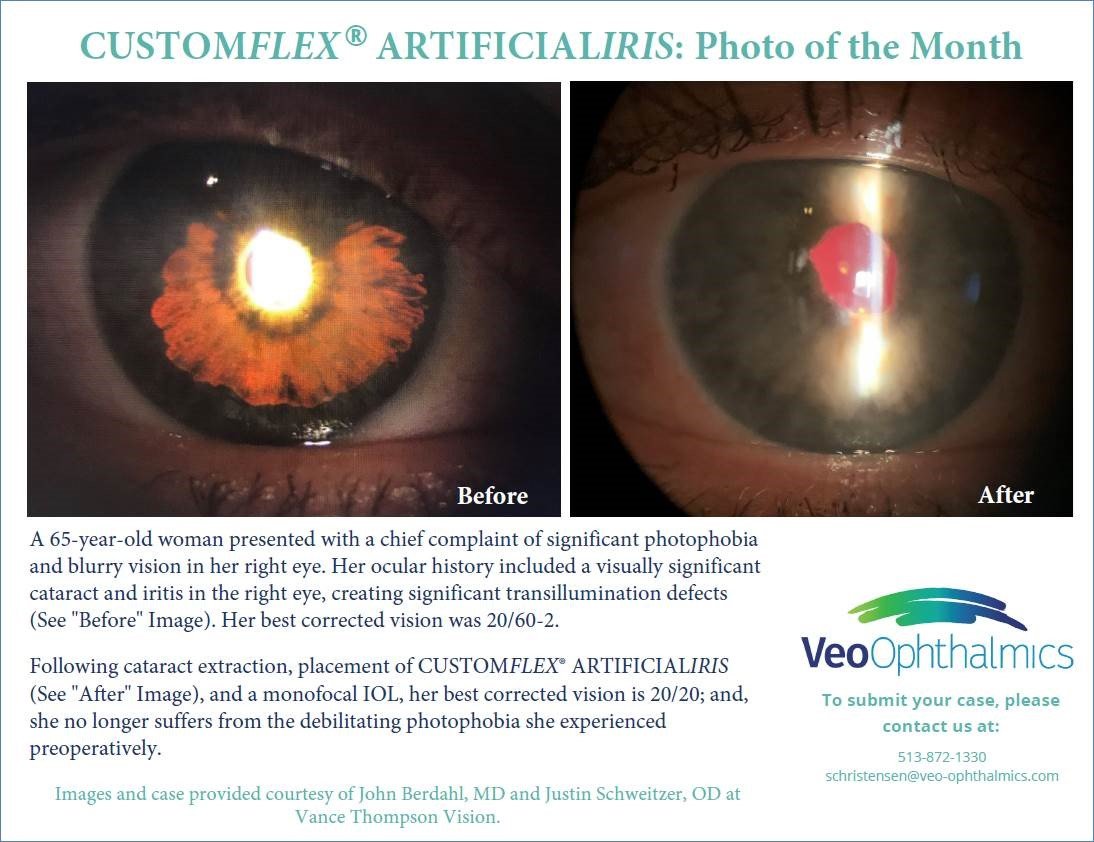
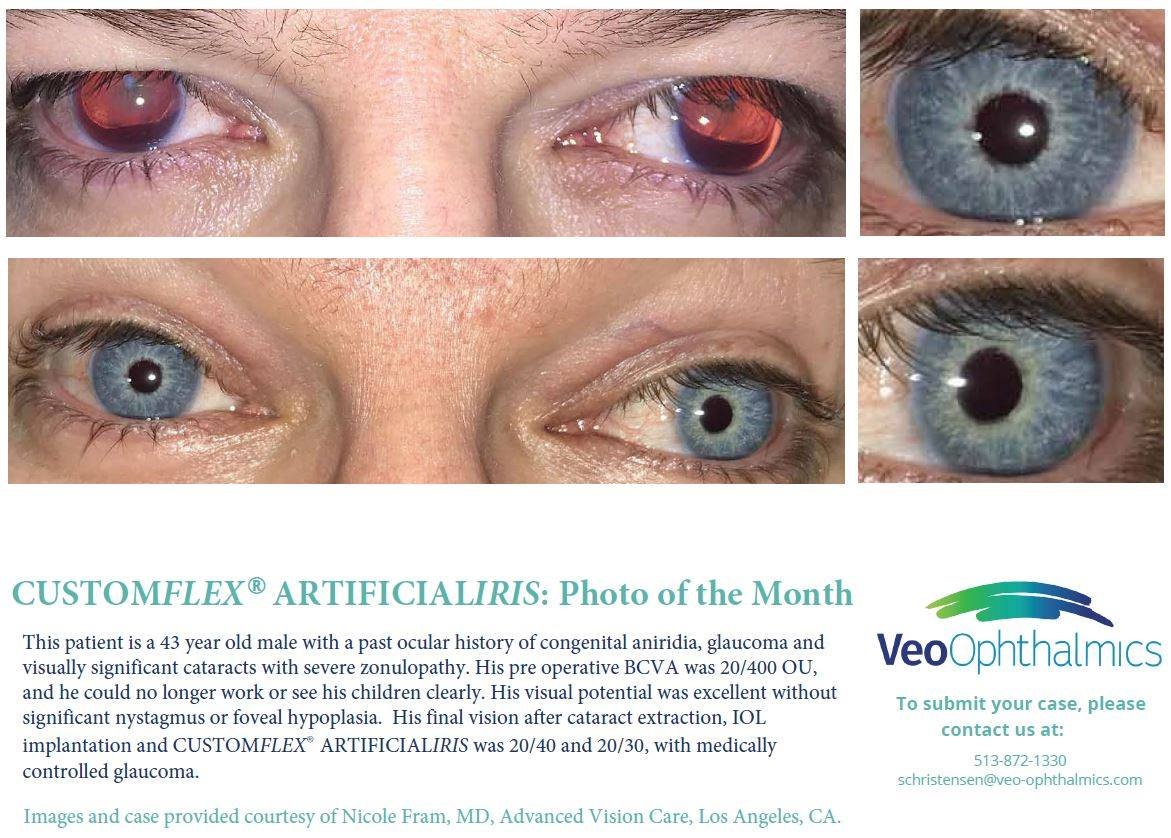
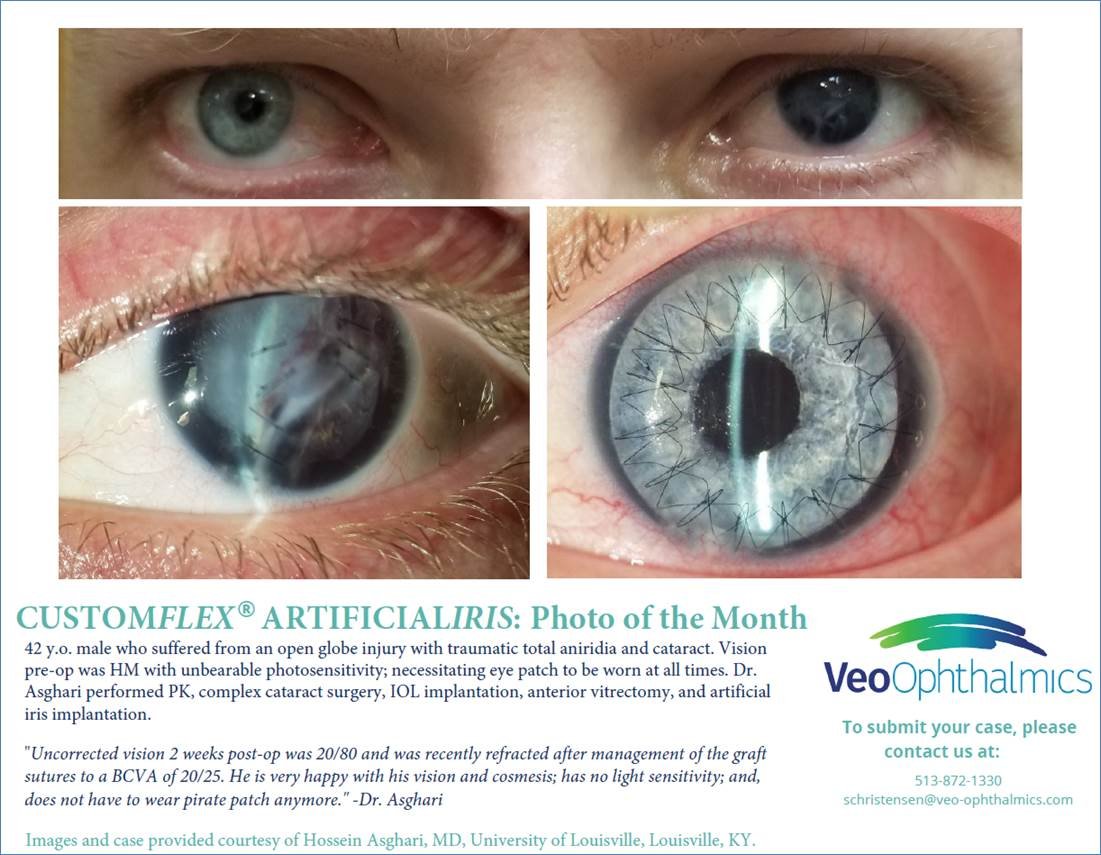
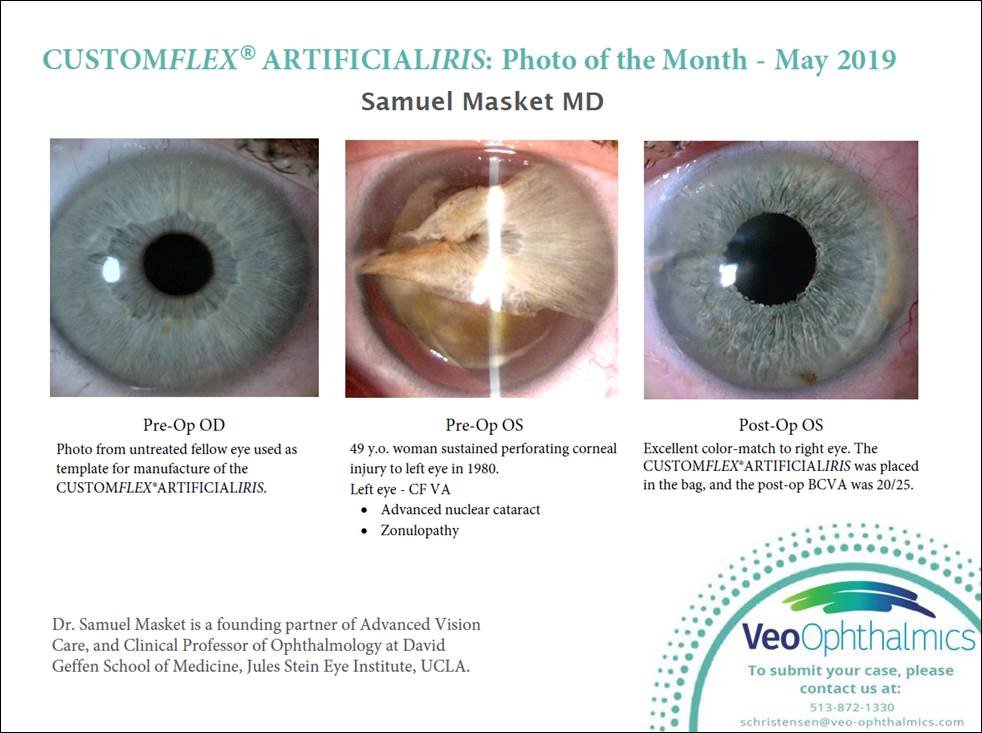
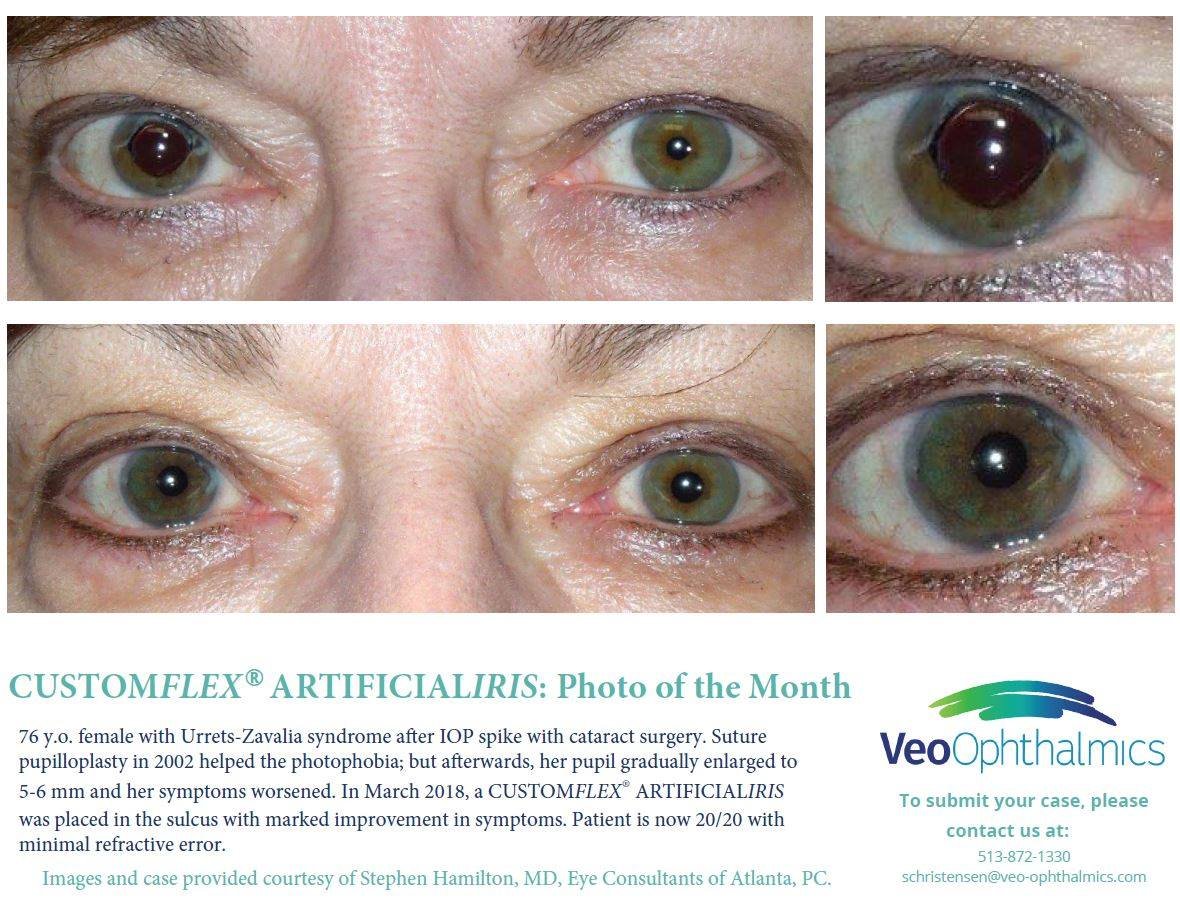
Before and after implantation of CUSTOMFLEX® ARTIFICIALIRIS
The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use for the treatment of full or partial aniridia.
“Approval of the first artificial iris provides a novel method to treat iris defects that reduces sensitivity to bright light and glare. It also improves the cosmetic appearance of the eye in patients with aniridia.”
Each CUSTOMFLEX® ARTIFICIALIRIS is custom-made to match a patient’s unaffected eye.
The CUSTOMFLEX® ARTIFICIALIRIS is intended for use as an iris prosthesis for the treatment of iris defects. The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use in children and adults for the treatment of full or partial aniridia resulting from congenital aniridia, acquired defects, or other conditions associated with full or partial aniridia. When implanted, the CUSTOMFLEX® ARTIFICIALIRIS mimics the natural iris and produces an improvement in the visual symptoms associated with aniridia that most affect the patient's quality of life.
Learn more about the CUSTOMFLEX® ARTIFICIALIRIS
ABOUT US
VEO Ophthalmics is dedicated to bringing innovative and quality medical devices to ophthalmic surgeons. Our primary focus is to provide quality devices for the ophthalmic surgeon which assist them in improving patient care. We do this by combining quality products and an exemplary customer service experience while strictly adhering to regulatory and quality standards. At VEO Ophthalmics, we believe that listening to our surgical customers and business partners is critical to our success.